On July 6th, 2023, the U.S. Food and Drug Administration (FDA) granted traditional approval to Leqembi (lecanemab, Eisai/Biogen), marking a significant milestone in Alzheimer’s research. This medication represents the first treatment to change the underlying course of the disease.
Alzheimer’s disease is a debilitating condition affecting millions of Americans, gradually eroding memory, thinking skills, and the ability to perform everyday tasks. Currently, over 6.5 million Americans live with Alzheimer’s, and its effects extend far beyond the individual diagnosed, impacting their loved ones as well.
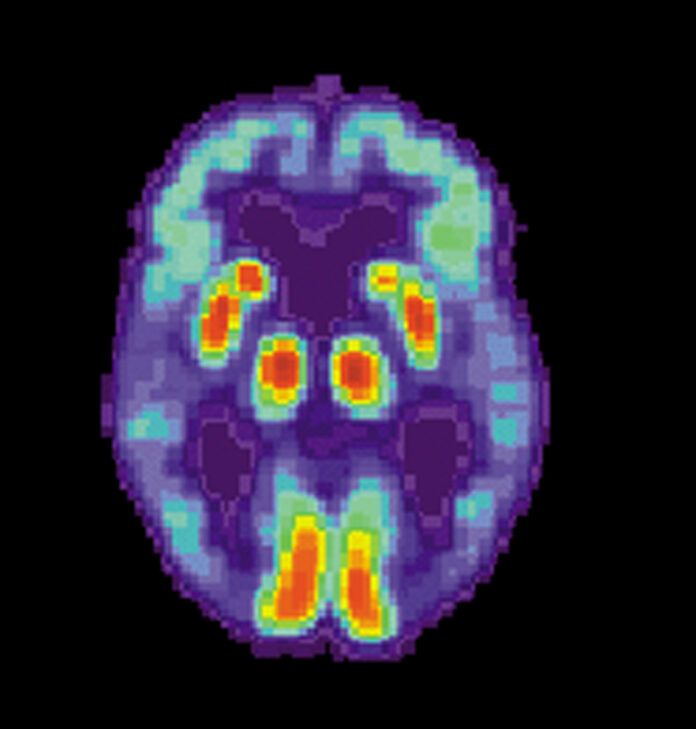
The specific causes of Alzheimer’s are unknown, but the disease is characterized by changes in the brain, including the presence of amyloid beta plaques and neurofibrillary tangles. These changes lead to the loss of neurons and their connections, resulting in cognitive decline.
Leqembi represents a breakthrough in treating Alzheimer’s disease as it directly addresses the underlying disease process rather than merely alleviating symptoms. The Director of Neuroscience at the FDA’s Center, Dr. Billy Dunn, emphasizes the significance of this approach. He states, “This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s instead of only treating the symptoms of the disease.”
Leqembi was approved under the Accelerated Approval pathway, a regulatory mechanism designed to expedite the availability of drugs for serious conditions with unmet medical needs. Researchers conducted a Phase 3 randomized, controlled clinical trial involving 856 patients with Alzheimer’s disease to evaluate the efficacy of Leqembi. The study followed a double-blind, placebo-controlled, parallel-group, dose-finding approach. The treatment was initiated in patients with mild cognitive impairment or dementia, confirmed by amyloid beta pathology.
During the trial, patients receiving Leqembi demonstrated a significant reduction of amyloid beta, which was both dose and time dependent. Notably, patients who received the approved dose of lecanemab, 10 milligrams/kilogram every two weeks, exhibited a statistically significant reduction of brain amyloid from baseline to 79 weeks.